«Evergreening»: How Roche's patent strategy drives up drug costs
Zurich, Lausanne, November 6, 2025
In brief:
An investigation on Roche’s patents related to Herceptin and its follow-on drugs for breast cancer shows that more than 100 patents still protect its products today.
This “evergreening” strategy has enabled the Swiss pharma giant to rake in over CHF 156 billion in sales and could allow it to maintain its monopoly until 2042.
- Public Eye calls on Swiss authorities to oppose this practice because it hinders access to more affordable biosimilars and prolongs overinflated prices.
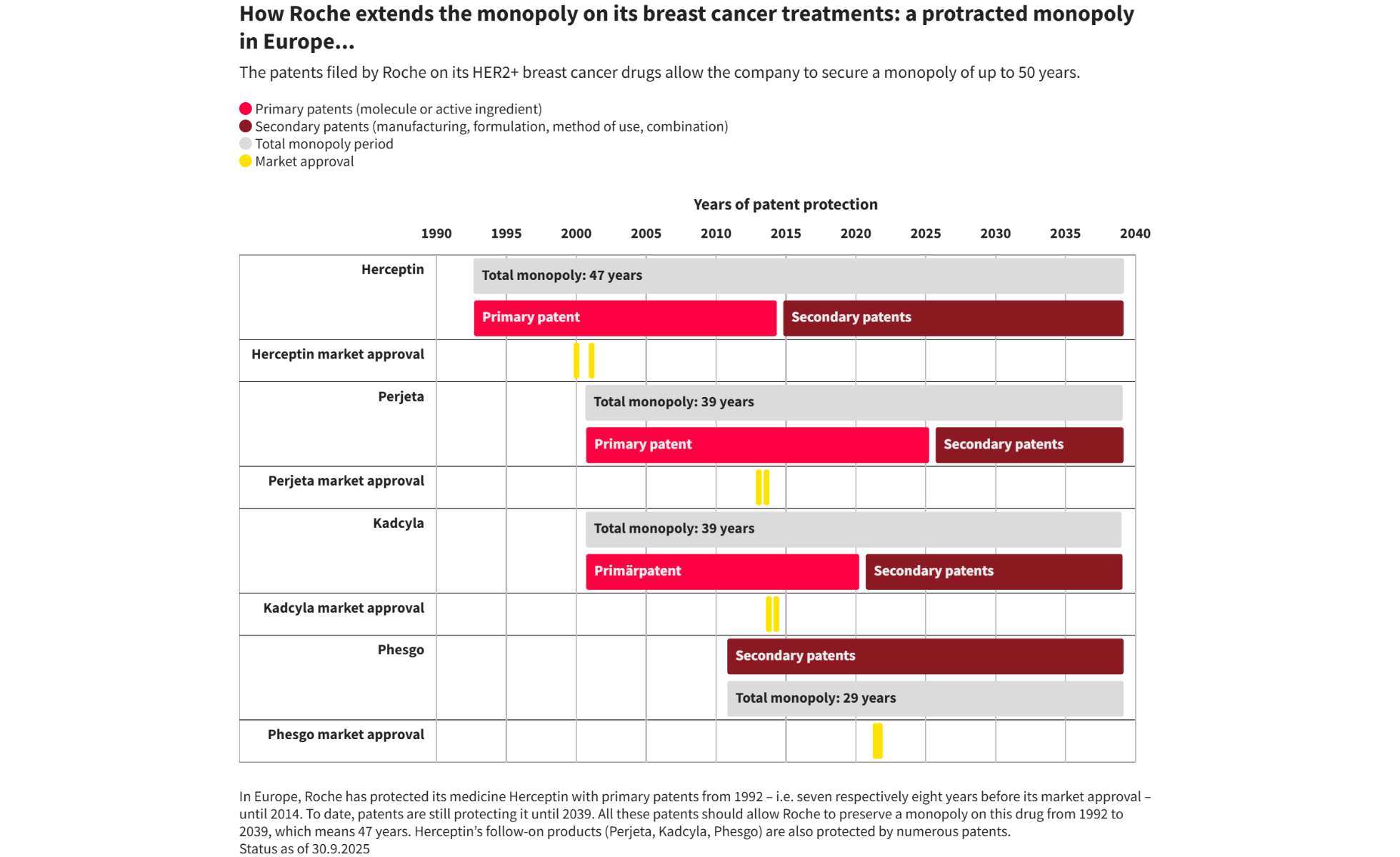
Since the late 1990s, Roche has generated sales of over CHF 156 billion with Herceptin and its follow-on products – Perjeta, Kadcyla and Phesgo – used to treat a particularly aggressive form of breast cancer (HER2+). These biological medicines, which are vital for numerous patients, constitute one of the Group’s main sources of revenue. But this success conceals a well-established “evergreening” – an abusive accumulation of secondary patents. Rather than enabling genuine therapeutic advances, they help to maintain an elevated monopoly price, to the detriment of patients and public health budgets.
Relying on official sources, Public Eye has identified 183 patents granted to Roche/Genentech in the US and 95 in Europe (also valid in Switzerland) to protect its antibody treatments. 100 are still valid overseas and 64 in Europe, which should enable the Group to retain its monopoly until 2042 or 2039 respectively. Almost 95% of these patents are not related to the active substance but to manufacturing processes, formulations or modified dosages. In addition, Roche takes legal action against its competitors, particularly in the US; seven claims were brought between 2017 and 2023 against manufacturers of Herceptin biosimilars for alleged patent infringement and one last August relating to Perjeta.
This strategy gives Roche powerful leverage to impose its excessive prices. In 2014, unhappy with the price set by the Swiss Federal Office of Public Health, it removed Perjeta from the list of medicines reimbursed by the compulsory health insurance scheme. This blackmail paid off for the company, as Perjeta was reintroduced a year later with a higher real price. This summer, Roche followed the same path for another of its cancer drugs, Lunsumio. As of early November, it still has not appeared back on the list.
Authorities in countries home to large pharmaceutical companies, like Switzerland, must urgently deal with the abusive proliferation of secondary patents on medicines. As a member of the European Patent Convention, Switzerland should request a more meticulous examination of patent applications. If it has allowed the question of exorbitant prices for patented medicines to be highlighted again, current efforts of the United States will not result in a real solution as the negotiations focus on the official list price – a fictitious reference price used for international comparisons – rather than the actual prices paid by health systems and patients. Even worse, the price of medicines in Europe and in Switzerland may increase in return if pharma companies follow through on their threats.
More information here or by contacting:
Oliver Classen, Media Director, +41 44 277 79 06, oliver.classen@publiceye.ch
Patrick Durisch, Pharma Expert, +41 21 620 03 06, patrick.durisch@publiceye.ch