The Paraquat Papers How Syngenta’s bad science helped keep the world’s deadliest weedkiller on the market
Laurent Gaberell (Public Eye), Crispin Dowler (Unearthed), March 24, 2021
Warunika was only 16-years-old when she took a swallow from an old bottle of Gramoxone weedkiller she found hidden on a ledge above a toilet in her family home. Her parents are sure she had not intended to die.
She was cooking eggs and fish for dinner when her hungry little brother came in, trying to grab things from her and hurry her along. They argued and she ended up hitting him with a broom. Their mother, Kumarihami, intervened, and while she was tending to the bump on her son’s head her daughter grew scared and “very silent”.
The next thing Kumarihami knew, Warunika had taken the bottle and poured some liquid into her mouth. Then she threw it at her mother and said, “here, I drank this.” “She did it to frighten me,” says Kumarihami.
Warunika died in hospital the following day.
 ©
Sachindra Perera
©
Sachindra Perera
The bottle she had grabbed was in her home because her parents were small-scale farmers, and at the time it was what they used to clear weeds from their few acres of rice fields in the northern central province of Sri Lanka.
Her father, Dharmasiri, says the bottle was old and had little left in it. “She probably thought since it was an old bottle it would not have been that potent.” But it had contained a concentrated solution of paraquat, one of the world’s most acutely toxic herbicides.
As little as 10ml - a tablespoonful - of Gramoxone can be fatal, and there is no antidote. Most people who swallow it do not survive.
At the time Warunika died, around 20 years ago, paraquat was causing hundreds of deaths a year in Sri Lanka.
No one knows the total number of people who have died from swallowing this chemical since the British company Imperial Chemical Industries (ICI) first put Gramoxone on the market in 1962. But according to one leading global authority on pesticide poisoning, University of Edinburgh professor of clinical toxicology Michael Eddleston, the figure must be at least in the tens of thousands.
At first, these deaths were concentrated in the UK and Western Europe, but they rippled out across the world as paraquat was introduced to new markets. In the mid-1980s, Japan was seeing more than 1,000 deaths a year. Countries including Sri Lanka, South Korea, Taiwan and China have all seen hundreds of deaths a year at times. Fatal poisonings have been recorded in places as diverse as the United States, Trinidad, Costa Rica, Malaysia, Fiji, Bangladesh, and India.
Many people - including many children - have died from accidentally taking a sip of paraquat that someone had stored in a drink bottle.
Many more have died in circumstances like Warunika’s - impulsive acts of self-harm that have more to do with a moment of stress than with any considered wish to die. In either situation, it is easy for a momentary mistake to be fatal, because the product allows for almost no margin of error.
Perhaps unsurprisingly, more than 50 countries have now banned paraquat. Sri Lanka itself phased out the weedkiller altogether from 2008, a few years after Warunika’s death. Announcing their decision, the Sri Lankan authorities said the death rate from paraquat poisoning was at 65% - higher than “any other agrochemical”. Other common weedkillers had death rates of between 4% and 8%.
 ©
Mark Henley / Panos Pictures
©
Mark Henley / Panos Pictures
Syngenta, the Swiss-headquartered, Chinese-owned agrochemical giant that inherited ICI’s pesticides business, continues to export thousands of tonnes of Gramoxone each year from its factory in the north of England - although the UK, Switzerland and China have all banned its use on their own soil. The company says paraquat is a “safe and effective herbicide when used as directed on the label”. It argues that almost “all modern innovations - buildings, bridges, railways, pharmaceuticals, automobiles, machines, and crop protection products - have been used for suicide” and that society needs to “focus on mental health issues, not deprive the world of useful technology”. It highlights how it has changed its packaging and labelling to discourage people from storing paraquat in food and drink containers, and trained “42 million farmers” in “proper use and storage”. Syngenta claims it has “helped address the problem of accidental ingestion” with the ‘safening’ agents it has added to Gramoxone since the 70s - a dye and an odour to warn people not to drink it, and an ‘emetic’ drug, to induce vomiting. It also claims product safety is “extremely important to us”, and that Syngenta has “led the continuous improvement of paraquat in the five decades since its invention”.
But now, a lawsuit in the US has unearthed an enormous cache of internal company documents which reveals how Syngenta and its predecessors knew for decades that the emetic in Gramoxone did little or nothing to prevent poisoning deaths - but continued to present it as effective to regulators and the public.
The documents show how ICI successfully used the addition of this drug to Gramoxone to help keep the product on the market at a time when it faced real threats of being banned in key markets; that it saw this patented additive as a way of blocking competition from other paraquat manufacturers; that the company continued with these strategies despite knowing it had no evidence the emetic would save lives at the concentration in which it was added; that it was repeatedly told by its own scientists that the amount of emetic in Gramoxone was too low to prevent fatal poisonings; and that it consistently resisted widespread introduction of safety measures like dilution because it did not consider them to be “economically acceptable” solutions to “the suicide problem”.
The documents show how ICI consistently resisted the widespread introduction of safety measures like dilution because it did not consider them to be "economically acceptable" solutions.
That this story can be told at all has much to do with the efforts of one man - a British scientist called Jon Heylings.
 ©
Unearthed
©
Unearthed
Heylings is a senior toxicologist, and an honorary professor of toxicology at Keele University, where for more than a decade he has run a successful company that offers specialist methods for safety testing chemicals without the use of animal experiments.
But, before that, he worked for 22 years at Syngenta and its predecessors, leading work to develop safer formulations of paraquat.
And he is now speaking out, for the first time publicly, to repeat what he first told his superiors at the company more than three decades ago - that he believes the Gramoxone Syngenta still sells in many countries is a lot less safe than it could be.
Heylings’ warnings are focussed on the emetic added to Syngenta’s paraquat products, a chemical codenamed PP796. The point of this additive is to reduce the product’s toxicity by causing people who swallow it to vomit out the paraquat before a fatal dose can be absorbed into the bloodstream.
But Heylings argues the amount of PP796 added to standard Gramoxone is far too little to trigger prompt vomiting in most people who swallow a ‘minimal lethal dose’ of the weedkiller.
He alleges this is because the concentration is based on a single “fabricated” internal report from 1976, in which an now-dead ICI toxicologist named Michael Rose manipulated data from a small-scale clinical trial to wrongly suggest humans were ten times more sensitive to PP796 than any of the three animal species it was tested on.
When Heylings first discovered the failings in the Rose report, in 1990, he documented his findings in a series of memos to his superiors. In these memos he advised that Rose’s work had “grossly misled” the business, that the concentration recommended by Rose was “probably well below an effective emetic dose in man”, and that a sharp increase in the concentration of emetic in Gramoxone could “reduce the number of fatalities attributed to paraquat poisoning”.
But, to this day, Syngenta still manufactures Gramoxone with the same concentration of PP796 it has had since the 70s.
 ©
PAN India
©
PAN India
Not only this, but the company has persuaded the Food and Agriculture Organisation of the United Nations (FAO) to adopt this concentration of PP796 as a global specification, in the agency’s guidance on the standards all paraquat-based weedkillers should meet.
When Heylings discovered in 2018, that the FAO was still using this standard, he again attempted to sound the alarm about the emetic, first in meetings and correspondence with Syngenta, then with the FAO and the US Environmental Protection Agency (EPA). “I have nothing against Syngenta,” he wrote in a 2019 email to the FAO. “I just want the next child that accidently takes a sip of paraquat weed-killer to have a fighting chance of survival by vomiting the poison out before a lethal dose is absorbed into the blood and they die of pulmonary failure.”
While Heylings was writing these emails, lawyers at the US firm Korein Tillery were getting ready to take Syngenta to court on behalf of a group of farmers who developed Parkinson’s disease after using paraquat to kill weeds. At some point in their investigation they found references to Heylings, and in March last year they flew to the UK to hear his story. Then, with his guidance, they combed through the decades-worth of paraquat documents Syngenta had been forced to release to them through discovery proceedings, and unearthed the material that told the full history of PP796.
When their case goes to trial next month, that history will be part of it. Stephen Tillery, lead counsel in the lawsuit, told Unearthed and Public Eye the “emetic issue” was part of his case because it demonstrated “the lengths to which this company will go to keep paraquat on the market”.
He added: "Paraquat was about to be banned in the 1970s because of the number of people who had died agonizing deaths from ingesting it. By claiming that the emetic would make paraquat safer to use they managed to keep paraquat on the market.”
Syngenta rejects Heylings’ allegations, and denies its decisions about the emetic were motivated by anything other than the desire to make paraquat safer. “While it may sound appealing on first encounter,” a Syngenta spokesman said, “Heylings’ argument that increasing the level of emetic improves the safety of the product is overly simplistic; the reality is complex and modern medical and scientific opinion does not support Heylings’ viewpoint.” He added: “We reject any suggestion that in developing this product Syngenta and its predecessor companies had any motive other than to find the most appropriate level of emetic in paraquat to best address the risk from accidental and deliberate ingestion.”
He added that the US EPA and the FAO had “not changed their recommendation about the emetic” since being contacted by Heylings. The FAO itself told Unearthed and Public Eye it had held a “special session” to review its paraquat specifications in response to Heylings’ concerns, and its report was “currently being finalised”.
For Jon Heylings, it is no small thing to find himself speaking out against the company he worked at for more than two decades. Heylings enjoyed his career at Syngenta, and counts many of his former colleagues as friends. But he is determined to see this through. “I want to be able to look back with my own grandchildren and say, I'm glad I did that,” he tells Unearthed and Public Eye.
“I know it took a long time to get the message through. I tried my best in the early 1990s, but I’m trying again now to convince Syngenta that they were wrong with the emetic concentration. And that’s what’s keeping me going.”
In 1986, when Heylings began working at ICI’s Central Toxicology Laboratory (CTL) in Cheshire, paraquat was big business.
According to CTL minutes from that time, the company was selling 15,000 tonnes of the chemical a year - enough to make about 75,000 tonnes of Gramoxone. Those annual sales were valued at around £200m - more than £600m in today’s money; they represented 30% of the company’s entire pesticide business and provided 30% of the profits. But the numbers of people dying from paraquat poisoning were putting those profits at risk.
The company estimated there were 2,000 deaths a year, with more than 95% thought to be suicides. At that time, up to 1,000 people a year were dying in Japan alone.
Unsurprisingly, ICI’s ability to keep making money from paraquat was under relentless regulatory pressure. Minutes from the company’s “Paraquat Strategic Action Committee” show ICI was not only fighting against efforts to ban or restrict paraquat sales, but also against regulators who wanted the company to dilute its high-strength liquid Gramoxone or replace it with solid granules.
ICI had already been making solid paraquat weedkillers for the UK market since the 70s, and it had evidence that they were significantly less dangerous than Gramoxone.
As internal reports explained, the granules were an “effective alerting agent” to prevent the poison being swallowed by accident. Company data also showed they made it harder to swallow large amounts of paraquat in suicide attempts. Gramoxone liquid was 20% paraquat, whereas ICI’s “Weedol” granules were just 2.5% paraquat, and 2.5% diquat, a related herbicide. On top of this, Weedol came in sachets which each contained less than a lethal dose. The consequence of these measures was clear, for example, from the unpublished findings of an ICI survey of UK paraquat poisonings in the early 80s: for the people poisoned with Gramoxone, the death rate was 78%; for people poisoned with granular paraquat products like Weedol, it was 16%.
But introducing solid granules more widely would have forced the company to invest heavily in “prohibitively expensive” new manufacturing facilities, and there was some concern about dust hazards.
Likewise, diluting the liquid - as it was eventually forced to do in places like Japan - added heavily to production and packing costs. As one typical ICI strategy document from 1987 shows, the company acknowledged diluting Gramoxone could produce a “measurable increase in the survival rate”, but claimed the product would need to be at least five times weaker to do so. Introducing that amount of dilution, or solid granules, “on a Global basis” would “destroy group profit from paraquat,” it states.

“None of the alternative formulations currently available offer an economically acceptable solution to the suicide problem either to ICI or to the farmer,” states a review of ‘safer formulation’ work from 1988. “[Significant] dilution leads to vastly increased formulation and packing costs, while the solid formulations available so far are prohibitively expensive and can still be used for suicidal ingestion.”
For this reason, company documents between 1985 and 1990 repeatedly state, it was not ICI’s strategy to “proactively” make changes to its paraquat formulations. Instead, less-toxic formulations were kept “on the shelf”, to be offered only to countries that were threatening to ban or restrict the chemical. The company did this because it considered the cost of introducing these products worldwide to be unacceptable, but also recognised bans were a major threat to its global paraquat business.
The 1987 document explained the strategy like this. There were “key features” of paraquat poisoning, it said, which “have tended to attract particular attention”. Among these were the fact that Gramoxone poisoning had just a 20% survival rate “compared with a more normal 80% survival rate for other chemical poisoning cases” and that at “low dose levels death is brought about through lung fibrosis and is usually prolonged and unpleasant”.
The “net result of these features” had been “the continuing presence over a long period of a strong media/regulatory lobby (often fuelled by deep concern within the medical profession) for the withdrawal of paraquat sales in many countries”.
 ©
Greenpeace
©
Greenpeace
ICI considered it had addressed its responsibility to “minimise accidental fatalities” with the dye, odour, and emetic that had been added to Gramoxone since the 70s. It did not accept that it had a responsibility to prevent the “social problem” of “suicidal abuse of paraquat”. But it knew regulators might take a different view. “We see no reason to change proactively from our current formulations,” the paper explained. “However the July 1985 Executive paper re-affirmed the need for a reactive strategy for formulation change given the existence of the ‘business’ problem brought about by suicidal/homicidal abuse of the product”.
The problem was that demands “continue to be made either for product withdrawal or for a change to ‘safer’ formulations in response to high levels of suicides”.
“ICI has continued to counter such lobbies with all necessary resource and efforts,” the paper stressed. “Nevertheless, in business terms it must be recognised that recent regulatory restrictions/withdrawals have led to a significant reduction in the Group profit generated by paraquat either through reduced sales or increased costs or both (eg Japan, Germany, Switzerland, Egypt, and now France from mid-1988).”
These pressures drove a search, within ICI, for ways to make Gramoxone less poisonous without heavily diluting the liquid or costing the company too much money.
The focus of this work, at the time Heylings joined CTL, was on attempts to create an emulsion of paraquat that would reduce the amount of poison absorbed by the body. CTL tested hundreds of paraquat emulsions. “What came to my attention as a scientist was that the formulations they were using all contained a much higher level of emetic, and they all contained a lower concentration of paraquat,” says Heylings. “And this made me think, you know, why is this, when the standard Gramoxone formulation that had been on sale around the world for the previous ten years or more contained a much lower level of emetic?”
Heylings dug into the ICI archives and found the Rose report from 1976.
The report’s author, a CTL toxicologist called Michael Rose, had recommended PP796 be added to Gramoxone at a rate of 5mg for every 10mls of liquid - or half a gram per litre. He estimated this amount would cause “the majority” of those who swallowed 10ml to vomit within an hour. Each of those numbers had significance. He chose 10ml because ICI thought this was the smallest amount of Gramoxone that could kill a person; he chose “an hour” - he claimed - because his tests on animals had shown PP796 could reduce the toxicity of paraquat if the animals vomited within that time. But his choice of 5mg was harder to explain.
Rose claimed this would be enough to cause vomiting in most people who swallowed the minimal lethal amount of Gramoxone. But all the experiments ICI had done with the drug on dogs, pigs and monkeys - which were summarised in Rose’s report - suggested you would need much more. Rose himself had also tested a mixture of PP796 and paraquat on dogs and monkeys, but he had used a vastly higher dose of PP796 in these experiments - the equivalent of 28 times the dose he was recommending for a 70kg man.
The explanation Rose gave for this discrepancy was that clinical studies had “indicated that man is more sensitive to the emetic effects of PP796 than the experimental animals studied”.

ICI had not originally developed PP796 as an emetic, but as a possible asthma drug. The chemical was rejected for this purpose by the company’s pharmaceuticals division, however, when the first clinical trials revealed “a variety of unpleasant side effects” including nausea, vomiting, and dizziness. The results from these early studies were the only information ICI had on the drug’s effect in humans, and Rose used these results to estimate a human emetic dose. So Heylings dug deeper, got hold of the original clinical trial data, and compared it to what Rose had written. To his surprise, he says, he “couldn’t reconcile the two sets of data”.
Heylings discovered Rose had based his recommendation largely on one tiny human volunteer trial in which just 12 people were given PP796, and only two of them vomited. Worse than that, he concluded Rose had cherry-picked the data, ignoring some volunteers who did not vomit while including participants from an entirely separate study, with the effect of exaggerating the drug’s emetic power in humans at very low levels. And, perhaps worst of all, Rose had ignored the fact that one of the two volunteers who did vomit only did so two hours after taking the drug - a duration that, even by Rose’s own standards, would be too long to help anyone who had swallowed paraquat.
Heylings first laid out his findings in a memo to his manager at CTL, toxicologist Lewis Smith, in January 1990. “Studies of poisoning cases involving emeticised paraquat formulations have not provided any definitive evidence that the introduction of 0.05% PP796 to paraquat concentrate in 1979 has resulted in a significant reduction in the number of fatalities attributed to the herbicide,” he wrote.

“This in my view, is not entirely surprising. My conclusion from studying the scientific evidence from clinical studies with the emetic is that the concentration of PP796 recommended in 1976 is probably well below an effective emetic dose in man.”
By this point, Heylings knew, ICI had done more-recent animal tests on PP796 which confirmed what the company had found in the 70s. Heylings wrote that all these animal studies were “in agreement” that 0.5mg per kilogram of bodyweight was the ‘minimal effective dose’ of the emetic. This dose is between six and eight times the dose of emetic an average human adult would get if they swallowed a tablespoonful of Gramoxone. In fact, Rose’s whole argument depended on one person who vomited after swallowing 8mg of the drug - a dose of 0.1mg/kg. That was the highest dose given to a human in any ICI study, and it was only given to that one person. And, as Heylings would note in later memos, Rose “completely ignored” the fact that the volunteer did not vomit until two hours later. Heylings concluded that, on the basis of the animal test data, there should be a “ten-fold” increase in the emetic concentration of Gramoxone, and said this would “reduce the number of fatalities attributed to paraquat poisoning”.
He was not alone in calling for a significant increase in the emetic. As early as 1985, Smith himself - who would later go on to become head of product development at Syngenta in Basel - was recommending a five-fold increase. Both men based their recommendations in part on lab studies run by ICI in 1985 which confirmed that, in dogs, 0.5mg/kg was the lowest dose that had any useful effect, but even higher doses were more effective. “By increasing the dose of emetic to dogs given paraquat, the dogs vomit more rapidly and severely and the concentration of paraquat in the blood decreases,” Smith wrote in a 1985 memo. By 1990, Heylings and Smith had seen enough evidence of the importance of this to advise the business, in a jointly-authored report, that the “time-to-vomit parameter is extremely critical” and in animals this had to be “within 20 minutes of ingestion in order to survive a lethal dose of paraquat”.
However, Smith was not keen to dig into the failings of the Rose report. This became clear when, in September 1990, Heylings again tried to revisit the issue, sending a detailed memo to Smith in which he laid out the data presented by Rose alongside the original clinical trial data, highlighting the information Rose had omitted or replaced. Smith only replied two months later: “It is clear from the data you presented that there was probably some misunderstanding or confusion in the way the case for the inclusion rate of 796 at 0.05% was arrived at. However, I am sure you will appreciate that attempting to reconsider the thinking and knowledge in 1976 when this decision was taken is extremely difficult. If my memory serves me correctly it was not even partly appreciated that the time to emesis in man that is required to prevent the absorption of paraquat is less than 30 minutes.”
Smith went on to say that he “and others in CTL, came to the view some years ago that it would be useful to increase the concentration of emetic in paraquat formulations”. There was “no disagreement” between him and Heylings that “an increase in emetic of 3-5 fold ought to be evaluated”.
But he did not want to go digging up the past. “I do not intend to pursue any further the reasons for the inclusion of PP796 at 0.05% as decided in the early part of 1976,” he wrote. Instead, he advised Heylings to focus on work to “evaluate formulations of paraquat that are intrinsically less toxic and contain increased concentrations of emetic.”
It would not be the last time Heylings would raise the issue.
Over the following half-decade, documents show, he raised his concerns in writing with various managers and experts in CTL and other parts of ICI, warning that Rose’s conclusions had “grossly misled” the business and pushing for an independent review of his findings.
He was not successful. But if Heylings had been allowed to dig deeper into the history, he might have learned that many of the problems he discovered in Rose’s work had been raised with Rose himself nearly 15 years previously.
In 1976, when Michael Rose began his work on the emetic, the heat on ICI’s paraquat business was even closer to home. Deaths in the UK from paraquat poisoning had been rising every year since the start of the 70s, and the company was under pressure to do something from newspapers, regulators and doctors. The reasons were illustrated, for example, in confidential ICI minutes of a 1973 meeting involving company executives and the toxicologist Roy Goulding, director of the National Poisons Information Service: “Dr Goulding felt that there was an urgent need to do something now to reduce fatalities not only from accidents but also from suicides. He believed the majority of people who try to commit suicide do not really wish to die and in these cases it was frustrating for a doctor not to have a specific treatment - ‘a hopeless therapeutic exercise’...
“He stated that in 1972 his centre had received 59 calls for advice on paraquat; six fatalities were recorded of which two were children. He reiterated his plea for ‘something to be done in a hurry’.” The company was also under “severe pressure” from regulators in other parts of the world, including Japan, Malaysia, and Western Europe, according to memos and board papers from the time.
But possibly the most serious threat to its business was the risk that paraquat could be banned in the US.
In mid-1975 the US Environmental Protection Agency (EPA) published rules under which pesticides thought to pose unreasonable risks could be put under intensive review to see if they should be banned. One of the triggers for this treatment - which was known as a “rebuttable presumption against registration”, or RPAR - was a lack of any emergency treatment or antidote for acute poisoning. In a letter to ICI in December that year, Chevron Chemical Company - the company which formulated and distributed ICI’s paraquat in the US - confirmed there was a risk paraquat would be placed on the RPAR list, and that there was a faction within the EPA that wanted to “‘get’ paraquat”.
The following month, Rose was asked to set up a working party to look into the “feasibility” of adding an emetic to the weedkiller. The US was at the front of his mind when he did. “Paraquat poisoning is causing the Company considerable concern,” he wrote in an invitation to the project’s first meeting, “particularly since the Environmental Protection Agency in the USA is currently questioning the safety of the product.” Rose knew a ban in a major market like the US posed particular risks, because it might lead other countries to follow suit. As he would later write to a CTL colleague in 1978, the company’s registrations of paraquat in the US and Japan were “‘threatened’ by toxicological considerations, particularly its use as a suicide agent. If the registration in the USA and Japan were to be withdrawn, the consequences to the Company would be very serious and the remaining markets in the rest of the world would be threatened.”

On 26 January 1976, Rose received a memo setting out ICI plant protection division’s “criteria” for any emetic they might want to add to Gramoxone. He was advised that “ideally it should be an established emetic agent” so that the company did not have to go through the “extensive toxicological testing” required for a new compound.
Rose held the first meeting of his working party three days later, on January 29, less than a month after first being asked to look into the issue. The meeting considered a short list of possible candidate chemicals: three well-known emetics, and PP796. The group decided the same day that PP796 would be the chemical assessed for use in Gramoxone. “It is of relatively low toxicity,” the minutes state. “Although it is not generally recognised as an emetic agent, there is considerable pharmacological and toxicological information on the compound.”
The idea of adding this particular chemical to Gramoxone was not new within ICI, but in the past it had been rejected. As early as 1971, for example, a scientist at ICI’s Industrial Hygiene Research Laboratory - what would later become CTL - had written to Dr PFC Bayliss at ICI Pharmaceuticals to ask for his views on the idea.
Bayliss’ view was important, because he was the author of all the clinical research on PP796 that Rose would later use to estimate the emetic dose in humans. Bayliss had replied that the chemical “although definitely emetic, does not have a clearly defined emetic dose, and therefore it would be difficult to choose a dose which could be guaranteed to produce vomiting in all individuals, unless it is a very high dose.” He added that even “when vomiting does occur, it is usually after 15 minutes; also by which time I would imagine that more than a toxic dose of paraquat will have been absorbed.” Bayliss was not alone within ICI in thinking an emetic would have to act very fast to have any effect on paraquat toxicity. As early as 1968, Dr AAB Swan, the head of IHRL and later CTL, wrote that centrally acting emetics were unsuitable for adding to Gramoxone because they “take at least 30 minutes to act as they have to be absorbed from the gut, and there is therefore time for dangerous amounts of paraquat or other pesticides to be absorbed as well.”

There is no evidence Rose concerned himself with these issues, although Swan was his boss at CTL and the man who gave him the emetic project. After the first meeting in January, the project moved rapidly through animal testing to Rose drafting recommendations for including PP796 in paraquat formulations. In August, ICI sent an update on the work to Chevron, advising that the British company intended to seek approval later that same year to start adding PP796 to Gramoxone on the UK market. The update stated that the dose added would be “5mg in 10ml of Gramoxone which is likely to produce emesis within 15 minutes in 80% of those ingesting such a quantity.”
In October 1976, a report went to ICI’s executive directors committee proposing worldwide introduction of the emetic - at this concentration - in its paraquat products.
But, at the same time, a toxicologist at Chevron’s Environmental Health Center called R. Cavalli was raising serious concerns about Rose’s work. At a meeting at Chevron Chemical Company’s research and development department on 4 October, Cavalli said he had reviewed the toxicology data on PP796 and there were “serious discrepancies between the actual data provided” and what ICI’s plant protection division (PPD) had been “telling us verbally.”
The minutes continue: “The data do not support PPD’s contention that 5mg of PP796 will produce emesis within 15 minutes in 80% of those ingesting such a quantity.” On 13 October Cavalli wrote to a colleague setting out in full all the vomiting data from the clinical trials of PP796. He noted this data could not support the claims about vomiting within 15 minutes, because “as far as I can tell, no one has vomited within 15 minutes”. He added that Rose’s favoured dose would be about 0.06mg/kg for a 77kg man, which was “significantly lower than the 2-3mg/kg found effective in the dog and monkey”. He concluded in terms that would be almost exactly echoed by Heylings 14 years later: “At CTL I was told that the compound was more active in humans, but the data does not support this.”
The following week Cavalli sent a telex to Rose himself, copying in various others at ICI, saying he was “concerned as argument for 5mg being an effective emetic dose in man is weak and still does not support the statement that it will cause emesis in 85% by 15 minutes”. He added: “I believe EPA will likely require actual data regarding effectiveness of dose in humans.”
Cavalli asked why Rose couldn’t just give some volunteers 5mg of the drug in a tablespoonful of water. Rose replied that the clinical data was “certainly weak” but a study on human volunteers was “not feasible for ethical reasons”. He added that “in the absence of hard evidence, I have produced a draft report making the case for addition at 5mg at 10ml” and had sent copies to Chevron. He concluded by suggesting he expected his argument to be enough to convince regulators on his side of the Atlantic: “We believe this case adequate for proposed European registration. Comments?”
The documents show that over the latter half of 1976 Rose and ICI moved from estimating 5mg of PP796 would cause vomiting in 80% of people within 15 minutes, to 70% of people within an hour, to, ultimately, “the majority” of people within an hour in the final version of his report.

Cavalli wrote back the following month, saying that Rose’s latest arguments would be “sufficient to send to the EPA with our first submission” but he still thought the agency might later want a “demonstration of the dose/effect relationship of PP796”. In April 1977, Chevron applied to the EPA to have PP796 approved for use in paraquat; its application stated: “Human clinical trials, supported by data from experimental animals, demonstrate that the amount of PP796 required to induce vomiting in the majority of humans ingesting it is 5mg."
In response to enquiries by Unearthed and Public Eye, a spokesman for Chevron Corporation said Chevron Chemical Company had ceased operations more than 30 years ago, and ceased distributing paraquat in 1986. He said that during the time it distributed paraquat, the company had pioneered many “product-stewardship programs” including “the first private poison-control hotline” and “the first child-resistant cap” He added: “During that time, ICI, the manufacturer of paraquat, elected to include an emetic in paraquat as an additional safety measure to further reduce the risk of accidental or intentional ingestion. Chevron Chemical Company submitted all required animal and human clinical data regarding paraquat to the United States EPA, which approved the emetic-containing formulation.”
Regardless of the weakness of Rose’s evidence, ICI hoped to persuade regulators across the world that “the new formulation is a major advance in our attempts to overcome the paraquat poisoning problem, because it effectively reduces paraquat’s toxicity” according to the strategy paper that went to directors in October 1976. But easing regulatory pressure was not the only objective. The company also wanted to use its patented emetic formulation to block competition from generic paraquat manufacturers. The paper advised that ICI’s overseas subsidiaries should begin talks with national regulators as soon as possible with the objective of ensuring “that the emetic is the sole paraquat formulation allowed to be sold”.
Within five years, ICI’s emeticised paraquat had been approved across Western Europe and in many other countries. And it had proven helpful in fighting off both regulator clampdowns and competition from Taiwanese paraquat manufacturers. A “Company Secret” memo from August 1981 titled “Emetic Paraquat: USA” describes how various countries had been persuaded to make the emetic mandatory.
“The requirement that paraquat products should contain an emetic has resulted in Gramoxone plus PP796 having an effectively exclusive position in the UK, France, Australia, New Zealand, Japan (except in the non-agricultural outlets) and Venezuela,” it states.
“Indeed in some countries (notably France and Venezuela) it is probable that the emetic has prevented paraquat from being banned.”

Probably ICI’s earliest success in using the emetic to keep paraquat on the market was in Samoa, a small Polynesian country known then as Western Samoa. In early 1977 the country’s medical authorities proposed a total paraquat ban, in response to “a rising rate of self-poisonings”, according to another secret ICI report, Paraquat Poisoning in Western Samoa, 1977-78. An ICI New Zealand medical advisor who visited the country at the time wrote that the majority of the deaths were people in their teens and twenties, and “a number of the deaths from suicide were emotional and immediate responses to a scolding by a parent and not calculated attempts to die." In response to the threatened ban, ICI medical representatives negotiated a deal with the Samoan government that “the ban would be rescinded on the assurance from ICI that all future shipments of ‘Gramoxone’ would contain the new emetic PP796."
In fact, the documents show, ICI had decided months before this deal to introduce PP796 to Western Samoa early on, because it thought the country would be a good place to test if the emetic worked in humans. An ICI executive explained this idea in a letter to Chevron Chemical Company in November 1976. “You may be surprised to see Western Samoa on the list of countries for early introduction,” he wrote. This was because “there has been a regrettably high incidence of suicides with paraquat there,” and ICI believed an early introduction in the country “would enable us to substantiate our belief that the new formulation will overcome the problem”.
By contrast, ICI had to wait until spring 1982 before the EPA finally approved PP796 for use in the US. Shortly afterwards, the EPA gave the company even better news, when it decided it would not impose an RPAR on paraquat. Explaining its decision, the regulator suggested it had been partly persuaded by Chevron’s arguments that adequate emergency treatment for paraquat poisoning did exist, but the emetic also had an influence. The EPA decision documents stated that the agency had now approved “an emetic which is to be incorporated into current paraquat formulations.” It added: “This emetic is intended to induce rapid vomiting thereby reducing the absorption of paraquat. The Agency, therefore, does not believe that adequate grounds currently exist for the initiation of an RPAR action based upon the lack of emergency treatment for oral exposure.”
But by the time the EPA finally made its decisions and paved the way for PP796 to be added to US paraquat, ICI already knew it had no evidence the emetic was making paraquat safer. By 1980, the company had given up on its experiment in Western Samoa, because of the difficulties it found gathering data from poisoning cases in the country. However, the limited data it did manage to gather offered no support for the idea that people swallowing emeticised Gramoxone were less likely to die.
Instead, the company switched its focus to the UK and Japan, in particular to a survey of UK paraquat poisonings it set up with the National Poisons Information Service at Guy’s Hospital in London. Here, too, the results were disappointing. So disappointing, in fact, that by mid-1981 there were real fears within the company that regulators would start asking for the evidence that PP796 was saving lives. The secret August 1981 memo Emetic Paraquat: USA explained the problem: “During the past year or more we have followed the effect of the emetic fairly closely in the UK and Japan. These observations have confirmed that PP796 is a highly effective emetic in man. However, no statistical evidence has emerged that the emetic has reduced the number of deaths with the product in either country… Although it remains difficult to discover all the facts surrounding such incidents, the conclusion that emerges from this scrutiny is that, at best, only a few people have survived paraquat poisoning because of the inclusion of the emetic. Even in these cases we cannot be certain that the emetic has contributed to saving life.
 ©
academia.edu / Andrew Revkin
©
academia.edu / Andrew Revkin
“The position that must now be taken on the effectiveness of the emetic formulation thus comes down to a belief that it may contribute to saving a small number of lives, all of them people who have swallowed a small amount of paraquat. Whatever ICI’s views on the subject, however, the outside world may independently reach a harsher conclusion.”
The report went on to warn that a “serious threat to our position could arise soon in some or all” of the countries that had imposed a legal requirement for paraquat to contain an emetic. “The registration authorities may wish to see evidence that the emetic formulation is helping to save lives… In some cases, it is even conceivable that an inability to demonstrate that the emetic is reducing the number of deaths from paraquat poisoning may lead to a ban on the product.”
The memo was also bleak about ICI’s prospects for continuing to use the emetic to gain advantage over competitors in the paraquat business. “In light of the current view of the probably small toxicological benefit which arises from inclusion of the emetic in paraquat, it is difficult to see how a case can now be made to registration authorities that an emetic should be included in all paraquat products, which is the means by which a commercial benefit is obtained from the emetic.” This was, it turned out, too pessimistic. In future years the company would succeed in persuading various countries to make an emetic mandatory. The memo’s assessment of the “toxicological benefit” of the quantity added to Gramoxone, however, would prove accurate.
A year later, in 1982, the ICI scientist who was overseeing the UK poisoning survey, TB Hart, wrote to colleagues confirming the gloomy picture of PP796’s performance: “There appears to be very little evidence to show that the addition of emetic to paraquat formulations has altered the mortality statistics overall,” he wrote.
Despite having ample evidence by the 90s that the emetic in Gramoxone was not saving lives, the company - which became Zeneca Agrochemicals in 1993 - continued to promote it with regulators, both as a means of fighting tougher regulation and of protecting itself against competition. In the mid-90s, the European Union was reviewing paraquat’s registration and Zeneca wanted to use the opportunity to make PP796 “mandatory in all paraquat formulations to be marketed within the EU”.
As part of this process, a manager at Zeneca Agrochemicals called Andy Cook, who is to this day a ‘global regulatory manager’ at Syngenta, drafted a report for the EU regulators on PP796. When the draft was circulated to CTL, Heylings was shocked to see it used the Rose report as evidence that the emetic concentration in Gramoxone was effective.
“Humans exhibit a greater sensitivity to the emetic response induced by PP796 than do either dogs or monkeys,” the draft claimed, citing Rose. “Extrapolating from data obtained in dogs and monkeys it can be concluded that a concentration of 5 mg PP796 in 10 ml of ‘Gramoxone’ (i.e. 0.05% w/v) should be added to the formulation in order to ensure that a person ingesting the lowest potentially lethal volume (10 ml) receives an effective dose of emetic.”
Heylings wrote back to Cook and others, repeating in detail the warnings he had given in 1990 about the failings of Rose’s analysis. He went on to cite more-recent lab studies done by CTL, which had found that dogs could tolerate up to five lethal doses of Gramoxone if the emetic was increased from 0.5g/l to 2.4g/l. He concluded: “In view of this background information, the rationale for including the emetic at a concentration of 0.5g/l in Gramoxone based on ‘greater sensitivity in humans’ is unsubstantiated.”
Despite Heylings’ warnings, the final document Zeneca submitted to the EU cited Rose’s report as evidence for its claim that 5mg of PP796 in 10ml of Gramoxone “should be added to the formulation to ensure that a person ingesting the lowest potentially lethal amount (10ml) receives an effective dose of emetic”.
Two years later, in June 1997, a Zeneca employee wrote to Cook to ask if “Zeneca would produce some sort of TO WHOM IT MAY CONCERN letter” confirming the emetic in Gramoxone met the FAO’s specifications. “The reason I ask,” she explained, “is because we are having trouble with [paraquat] in Nigeria at the moment, where it is wavering towards a ban, and currently a big tender has come up from the oilfields where we want to submit GRAMOXONE - big bucks if we get it.”

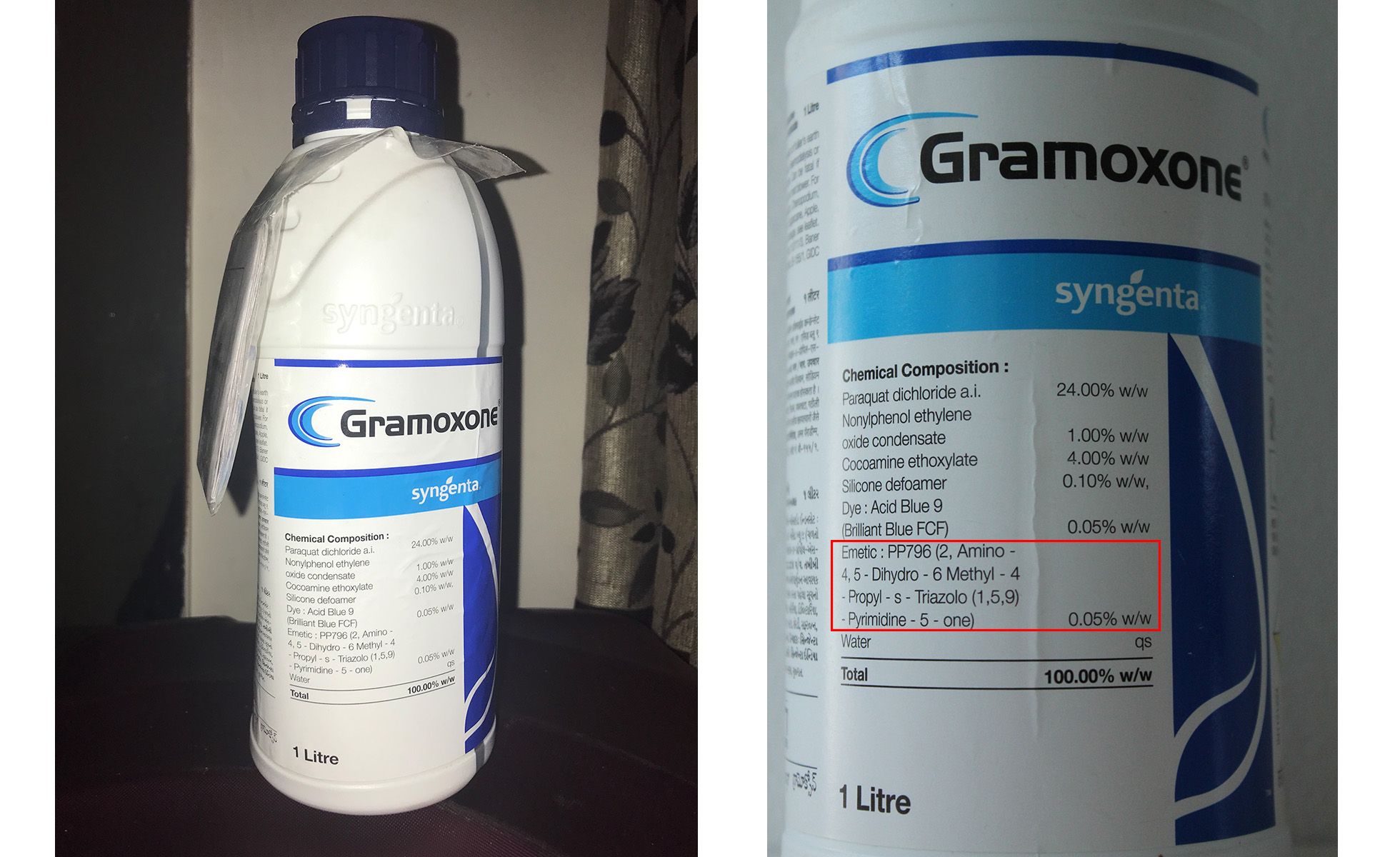
The FAO had just published specifications that required paraquat to contain an effective emetic. Its criteria were, in fact, already based on Zeneca claims about PP796, although they did not mention the chemical by name. They included the requirement - which is still in the FAO’s current specification - that the emetic added must cause vomiting “in about half an hour in at least 50% of cases”. Cook wrote to Bob Scott at CTL asking him to produce a short document “for use opposite regulators (mostly in [least-developed countries])” which “clearly establishes that PP796 fulfills the criteria” set out by the FAO. He added that it would need to be ready in time “for use with Nigerian regulators visiting the UK” that September. In the same file of documents, Unearthed and Public Eye found a TO WHOM IT MAY CONCERN letter - unsigned and undated - which claims that PP796 is “an extremely potent oral emetic, with man particularly sensitive to its action”. It goes on to cite the same clinical studies as Rose, as evidence that half a gram of the emetic in a litre of Gramoxone is “likely to cause vomiting” in those ingesting 20ml or more.
In 2003 - as Zeneca had intended - the EU re-approved paraquat and made it mandatory for all liquid paraquat products to contain an “effective emetic” that took “account of the FAO specification”. In the same year, the company - which had become Syngenta in 2000 - succeeded in persuading the FAO to name PP796 in its specification as the “only compound” found to meet the requirements for a paraquat emetic. In June 2003, a Syngenta employee summarised his meeting with the FAO by saying: “We appear to have met all our goals… PP796 will be mentioned by name, but the FAO will leave scope in the wording to allow future inclusion of other compounds”. The new specification also decreed that PP796 should be added to Gramoxone-type solutions at a ratio that was the same as that found in the standard emeticised Gramoxone ICI started producing in the 70s.
But at the same time Syngenta was pushing the FAO to adopt this level of PP796 as a global standard, there were some within the company who were worried about drawing too much attention to the contribution this additive actually made to safety. In May 2003, a global regulatory manager at Syngenta wrote to colleagues to warn that Bayer, one of Syngenta’s competitors had shown interest in adding PP796 to insecticides containing the chemical aldicarb. This he said raised “a number of potential concerns” including the “potential to focus unhelpful attention on the specific contribution of the emetic rather than the combination of safening agents in Gramoxone”. In addition, he warned, it could “focus increased attention on the specification and regulatory package for PP796 supporting its inclusion in Gramoxone,” and it might “trigger increased scrutiny of the inclusion level of PP796 relative to [the toxicity] of paraquat”.
Heylings’ work in the 90s focused on ways to reduce paraquat’s toxicity by increasing the emetic, in combination with other additives intended to slow the body’s absorption of paraquat, giving the emetic more time to work. Early on, he experimented with options that included five times the emetic as standard Gramoxone.
However, he found there was resistance to that level of increase from some commercial players in the business, because of the high cost of emetic.
That concern was illustrated in an April 1990 memo he received from a manager in the company’s herbicides business, about a new formulation he was working on. “I’ve done a rough costing of your latest ‘Magnoxone’ formulation,” the manager wrote. Paraquat itself, the memo showed, cost ICI £3.50 a kilo to produce; PP796 cost £30 a kilo. Heylings’ recipe would have increased the amount the company spent on emetic from 1.5 pence a litre to 7 pence a litre. “As you can see,” the manager wrote, “the major component of variable cost increase is PP796… If we are to switch any significant volume markets to ‘Magnoxane’ we would need new manufacturing facilities for PP796.” In a discussion paper circulated to the Paraquat Strategic Action Committee that October, the same manager warned that any “significant increase in emetic concentration would face a high financial penalty.”
The focus of Heylings’ work shifted to mixtures with three times the emetic, plus other ‘safening’ ingredients.
Syngenta had high hopes for this new product, which it internally dubbed “the Prometheus technology”. A “highly confidential” briefing on the project from 2001 shows the company saw it as “a unique opportunity to improve key stakeholder… perception of Gramoxone - an improved brand image”. Syngenta was targeting a tenfold reduction in acute oral toxicity to humans, although it knew that “at best” this might improve the survival rate for self-poisoning “from 25% to 50%”. And it hoped the new safety measures would allow it to move back to high-strength products in places like France, Germany and Japan, where it had been forced to dilute its liquid paraquat products. A 2003 strategy document explained that - as with PP796 in the 70s - once the new product was approved Syngenta would “seek to gain legitimate advantage” over competitors “by establishing this product as the new minimum standard for paraquat.
Inteon was trialled from 2004 in Sri Lanka, where paraquat was at the time responsible for 400 to 500 poisoning deaths each year. A Syngenta-funded study of this trial was later published, which found that that the new formulation improved three-month survival rates from 27.1% to 36.7%, and saved an estimated 30 lives over the course of the study.

Syngenta wrote to the US EPA in September 2006 trumpeting the results of the Sri Lanka study as “new scientific evidence” which “demonstrates that Inteon works as predicted and reduces human deaths following ingestion of paraquat”. In the same letter, the company stated that the Sri Lanka data showed a “significant increase in human survival compared to the non-Inteon paraquat”. On this basis, the company explained, it wanted the EPA to cancel the registration of the Gramoxone formulation it had previously sold in the US, saying “the safer formulation is already established in the [US] marketplace, thus ensuring significantly greater protection of human lives”. The company also opposed demands of other manufacturers that they be allowed to “introduce their versions of the old paraquat formulation” because this would “unreasonably and significantly diminish human safety”.
But the improvement seen in the study still left Sri Lanka with a product that killed well over 60% of those who swallowed it.
This was not good enough for the Sri Lankan authorities. In 2008 they began phasing out paraquat; in 2014 it was finally banned.
Meanwhile, Syngenta was also having trouble with its efforts to persuade the EPA of the benefits of the new product. The company had initiated a new series of tests on dogs, comparing Inteon with both Gramoxone and the extra-high strength Gramoxone available on the US market. To Syngenta’s surprise, these tests found that the older paraquat formulations were less toxic at certain high doses than Syngenta had previously assumed. This, in turn, meant the safety improvement shown in dog experiments with Inteon was less than previously assumed. According to a later Andy Cook email to Syngenta colleagues, the company took the decision to “terminate the ‘Inteon’ project” in the first three months of 2008 “after commercial launch in several countries”. He said that while studies in Sri Lanka and South Korea “provided some limited evidence for improvements in survival it had become clear that the improvement in safety was considerably less than the anticipated (and repeatedly claimed) 10-fold and no longer met the company’s objectives established for the project".
The internal documents obtained from Syngenta by US lawyers include a “Standby Statement” the company prepared in April 2008 with Q&As to use with regulators and the press about its Inteon findings. “Because the reference benchmark for Gramoxone formulations has changed, we are not able to conclude that there is as significant and consistent a safening effect over conventional Gramoxone formulations as originally expected,” it states. “However, it is prudent to assume that the degree of safening of INTEON formulations is consistent with the published results of a monitoring survey in Sri Lanka which showed an approximately two-fold reduction in toxicity.”
After Inteon was eventually pulled from the market, it was replaced in the US by a new formulation of Gramoxone with roughly three times the level of emetic as ‘standard’ Gramoxone - 1.5g PP796 and 240g paraquat.
Elsewhere, in countries like India, Syngenta carried on selling ‘standard’ Gramoxone, with the same ratio of emetic to paraquat it had used since the 70s.
In August 2018, Jon Heylings emailed Andy Cook, who was by that point Syngenta’s “global regulatory and product safety lead” for paraquat and related pesticides, to describe how he had come across the FAO’s latest specification for PP796. “This 0.23% ratio remains to this day an ineffective emetic concentration if a minimally-lethal dose of paraquat… is ingested in man. For the reasons I have described above, this should be ten-fold higher… The FAO spec goes on to describe how ‘emesis must occur within 30 min in at least 50% of poisoning cases’. Since the PP796 dose would be below threshold for causing vomiting in a minimally-lethal paraquat dose, this 0.23% PP796 would be completely ineffective.”
This began nearly a year of meetings and correspondence between Heylings and Syngenta, in which he laid out in detail his allegations about how Rose had manipulated the clinical data, and how he had made various former and current company employees - including Cook - aware of his concerns in the 1990s. Meanwhile, emails circulated among Syngenta staff in Switzerland, the UK and the US about how to respond to Heylings, an internal briefing was prepared about Heylings and his allegations, and a Syngenta statistician was commissioned to re-analyse the clinical data Rose had based his recommendations on.
This culminated in a letter sent to Heylings in May 2019 by Dave French, the company’s global head of regulatory affairs. “You have raised concerns about the data from an analysis of clinical trials reported in an ICI 1976 research report, which you are concerned cannot properly be used to support the level of emetic in Syngenta’s paraquat products because it was fabricated.
“There is no evidence of fabrication associated with the 1976 research report and simply no basis to believe the author would have reason to fabricate results.”
French went on to quote in technical detail from the findings of a new analysis Syngenta had conducted of the Rose report data, to show how individual steps in Roses’ analysis might have had innocent or justifiable motivations. “ICI and Dr Rose had no conceivable motivation to falsify or fabricate this 1976 analysis, the voluntary actions of the company were clearly directed to improving survival,” he concluded.
However, the actual report by Syngenta’s statistician confirmed something Heylings had been saying since 1990 - that the clinical studies Rose had used did not provide enough evidence to determine the right dose of PP796. Even if you ignored the time these volunteers had taken to vomit, the report concluded, “the clinical data are incapable of supporting a confident conclusion on the appropriate inclusion level of PP796 in paraquat formulations”. If you were trying to work out the right dose to cause vomiting within an hour or 30 minutes, it added, “the uncertainties increase further.” But this did not matter, Syngenta argued, because Rose’s work had been “superseded” by human poisoning studies that had tracked the effect of the emetic in real situations.
“In any event,” as French’s letter put it, “this 43 year old study has long since been superseded by later studies of human ingestion incidents reviewed by global regulators.”
In particular, French indicated, Syngenta’s evidence that Gramoxone met the FAO’s specification did not come from the Rose report, but from human poisoning data published in a 1987 paper called Treatment of paraquat poisoning in man: methods to prevent absorption, written by two eminent poisons experts called TJ Meredith and JA Vale. “The Meredith and Vale publication reports that, overall 65% of those drinking paraquat formulation containing the emetic vomited within 30 minutes and, with respect to accidental poisoning where lower volumes were ingested, 55% of those consuming <2g paraquat ion vomited within 30 minutes.”
In fact, these figures came from the survey of UK paraquat poisoning patients that ICI funded between 1980 and 1982, shortly after the emetic was first added to the company’s paraquat products. It was this survey that had caused dismay within ICI at the time when TB Hart - the ICI scientist overseeing it - reported to colleagues that there was “very little evidence to show that the addition of emetic to paraquat formulations has altered the mortality statistics overall”. The full report of this ICI study was never published, and indeed the figures taken from it are explicitly described in the Meredith and Vale paper as adapted from “unpublished data”.
But Unearthed and Public Eye have reviewed a full copy of the unpublished 1982 report, Paraquat poisoning in the United Kingdom by Amanda Bramley and TB Hart.
The report reveals that most of the people in that survey who swallowed emeticised paraquat did not take Gramoxone at all - but rather Weedol or Pathclear, the low-strength granular paraquat that was available in the UK at the time.
And of the cases where people took less than 2g paraquat and vomited within 30 minutes, almost all were Weedol.

Unearthed and Public Eye shared these documents with Michael Eddleston, a professor of clinical toxicology at the University of Edinburgh and one of the world’s leading authorities on pesticide poisoning. Asked if the Meredith and Vale paper provides evidence that Gramoxone met the FAO specification, he replied, “Obviously it does not.” First, he explained, it was not “primary data”, but rather “someone reporting on what someone else did”. Second, it was “not clarified in that paper at all” that the majority of patients were ingesting Weedol, which had a different formulation and toxicity to Gramoxone. In each case where a patient had vomited after swallowing Weedol, “it tells you nothing about Gramoxone”.
French’s letter to Heylings offered a third argument, which was reiterated by Syngenta in its response to Unearthed and Public Eye’s investigation - that increasing the amount of emetic in paraquat mixtures might actually make them more dangerous. “Medical opinion has evolved in the thirty years since Heylings first worked on this product,” a Syngenta spokesman told Unearthed in a statement. “Today, eminent medical experts advise against high emetic levels based on concerns that they can increase toxicity. In addition, emetic-induced vomiting of paraquat can cause additional damage. Several studies indicate little correlation between higher added emetic levels and improved outcomes in deliberate ingestion cases.”
The company did not comment on why it markets formulations with significantly increased levels of emetic in some markets, such as the US, and not others, such as India. When Unearthed and Public Eye put the firm's argument to Michael Eddleston, he noted there was no greater hazard than death. Given that Gramoxone killed large numbers of people who swallowed small amounts, the only way increasing the emetic could increase the risks it posed was if it caused more deaths. This, he concluded, was very unlikely.
Eddleston also took issue with the idea - voiced frequently over the years within Syngenta and its predecessor companies - that people who commit suicide with paraquat always ingest such large amounts that an emetic could not be expected to save them. Syngenta’s own Inteon study in Sri Lanka - of which Eddleston was an author - more than half the patients reported swallowing less than 30ml, or three tablespoonfuls of liquid. And for people swallowing these quantities, the death rates were lower for those swallowing Inteon than those swallowing Gramoxone.
And for people swallowing these quantities, the death rates were lower for those swallowing Inteon than those swallowing Gramoxone. In the case of those who swallowed 10 to 30ml, the death rate was reduced from 78% to 55%. This study and the ICI animal studies suggested increasing the amount of emetic in Gramoxone would save some lives, he concluded. How many was unclear.
“Very many people who become a suicide are simply self-harming with a lethal compound,” Eddleston said. “It is simply like having a gun in your house compared to having paracetamol in your house.”
Eddleston has seen “tens, maybe hundreds” of people die from pesticide poisoning. With paraquat patients, he said “many of them live for several days after and they can talk to you, tell you what happened. And often these were very spontaneous, they had no intent to die at all. They would drink the cap of a bottle, have a sip.”
Asked if paraquat could be made safe, Eddleston replied that you could “make it safer, but I don’t think you can make it safe”. In Sri Lanka, in the period before paraquat was banned, manufacturers were made to dilute products - including Inteon - from 20% down to 6.5%. Eddleston said that in this period the mortality rate fell from 65% to 20%. “Is [that] safe?” he asked. “I feel like if you are using these pesticides in rural communities without adequate protection, without adequate storage facilities, and in stressed communities where drinking this is simply a response to stressful situations… I think it has to be safer than that. I don’t think that’s safe.”

In Sri Lanka today, deaths of this kind are a rarer event, according to Dr Shaluka Jayamanne, a lecturer at the faculty of medicine at the University of Kelaniya. He started his research in toxicology in the early 2000s, when he was working as a doctor in the general hospital in the city of Polonnaruwa.
At that time, he says, a third of the hospital’s bed occupancy was people with pesticide poisoning, and “the wards were heavily overcrowded, so we had to look after two patients in one bed”. Since then, Sri Lanka has not only banned paraquat, but also the most toxic organophosphate insecticides. Those chemicals, he says, were the “main causes of pesticide-related deaths in Sri Lanka, and with the bans pesticide-related deaths have come down drastically”. The burden on the hospitals has also “drastically come down”. But in other parts of the world, doctors have continued to face waves of paraquat poisoning.
In September 2019, not long after French sent that final letter to Heylings, a group of doctors at Veer Surendra Sai Institute of Medical Science and Research, in Odisha, India, went on a 24-hour hunger strike to demand a ban on paraquat sales. Newspapers reported that 177 patients had been admitted to their hospital with paraquat poisoning in the preceding two years, and 170 of them had died.
Speaking to Unearthed and Public Eye in 2021, one of the doctors who led that protest, Dr Shankar Ramchandani, says the Government of Odisha had since restricted the use of paraquat but “patients are still coming now”.
“A ban is the only solution,” he says.
Asked to describe how he and his colleagues came to campaign against paraquat, Ramchandani describes how he had treated a man in his thirties who had swallowed a small amount of the weedkiller after a family dispute. He was having respiratory problems and the doctors tried to manage him in intensive care, but he did not survive. Ramchandani recalls how the man's wife offered him her gold chain in an effort to persuade the doctor to save her husband. “That was a very difficult situation for all of us,” he says. “That started the movement in my heart. So we started our movement.”
For Ramchandani, the situation is explained in terms that would have made sense to the toxicologist Roy Goulding in 1973, or to Shaluka Jayamaha or Michael Eddleston in 2021, or, no doubt, to any number of doctors who have watched people die of paraquat poisoning in the intervening decades. “We are seeing lots of patients dying, and we are unable to do anything because there is no antidote,” he says. “It is very painful for a doctor that patients will die in front of him and he cannot do anything about it.”